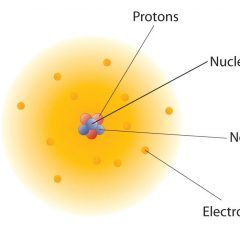
Why, in an atom, does the negatively charged electron not collapse into the positively charged nucleus?
If the nucleus of an atom is positively charged then why doesn’t the negatively charged electron collapse into it? To answer this we’re going to have to delve into the history of how our understanding of the atomic world has evolved, because the very nature of this question is based on a very old view of the atom. In the Beginning … When Ernest Rutherford, the New Zealand-born founder of nuclear physics, first...
An Uncertain Future – Predicting Movements of Objects
We like to think that science has all the answers and that the more we learn the more we’ll be able to predict and control our environment. But what if there was a limit after which there’s too much uncertainty.
Chaos and Disorder – or Why does it always happen to me ?
Why do so many things seem to go wrong ? Sometimes you suspect that the universe is out to get you! We need to look at some scientific theories surround something called Entropy or the “The laws of thermodynamics”