Why is the Earth’s Core Still Hot ?
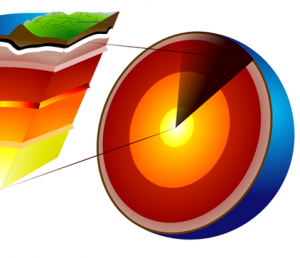
We know that the Earth’s core is hot, but not how hot. Actually, even geologists don’t know exactly what the temperature is, but they do know that it is almost as hot as the sun. Now that’s hot !
The Why
The surface is losing heat by being in contact with cooler air. But the core is continually heated by the decay of radioactive elements. Scientists believe that the original heat from the formation of the Earth is still being played out in these transformations. The sun is likely to expand and burn us before the core of our planet cools off.
Guestimating it’s Temperature
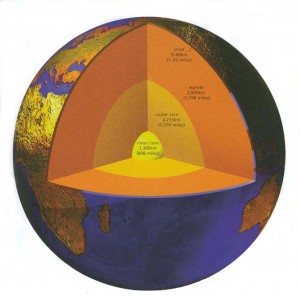
The rule of thumb has traditionally been that for every 33 m in depth, the temperature of the Earth increases by 1C. But this old approximation was based on distances that scientists could measure by drillholes. If this ratio held true all the way to the core of the Earth, its temperature would be 100,000C. However most geologists agree that the actual temperature is closer to a still unfrosty 5000C.
Fortunately for us the amount of heat that reaches the Earth’s surface is not enough to affect our climate drastically, and what heat there is quickly radiates into outer space. However the heat transfer is not constant everywhere. Just as the geothermal gradient varies from place to place, so does the heat flow, which is greatest near young volcanoes and active hot springs, and least where the crust is oldest and least active.
So what’s going on down there ?
The Earth’s crust helps insulate the core from losing more heat. These cracks in the crust, like volcanoes and springs, are the few venues that allow us to glimpse the heat trapped in the sizzling core below.
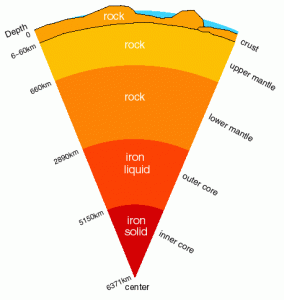
The Earth is thought to have formed from the collision of many rocky asteroids, perhaps hundreds of kilometres in diameter, in the early solar system. As the proto-Earth gradually bulked up, continuing asteroid collisions and gravitational collapse kept the planet molten. Heavier elements – in particular iron – would have sunk to the core in 10 to 100 million years’ time, carrying with it other elements that bind to iron.
Gradually, however, the Earth would have cooled off and become a dead rocky globe with a cold iron ball at the core if not for the continued release of heat by the decay of radioactive elements like potassium-40. uranium-238 and thorium-232, which have half-lives of 1.25 billion, 4 billion, and 14 billion years, respectively. About one in every thousand potassium atoms is radioactive.






